
Vol. 40, n.º 1, 2007
REVISTA
ESPAÑOLA DE
Vol. 40, n.º 1, 2007 |
CASUÍSTICA
Jesús Vera Álvarez1, Miguel Marigil Gómez1, María Dolores García Prats1, Manuel Abascal Agorreta1, José Manuel Ramón Cajal2, José Ignacio López López3, Juan Pablo Royo Goyanes2
Hospital San Jorge, Huesca.
1 Servicio de Anatomía Patológica.
2 Servicio de Ginecología.
3 Servicio de Radiología.
jjvera@salud.aragon.es
SUMMARY
One case of extraovarian primary peritoneal carcinoma is reported. This rare interesting tumor is characterized by a peritoneal carcinomatosis with ascites and by a histological pattern similar to ovarian serous papillary carcinoma, but it either spares the ovaries or involves the surface of the ovaries only microscopically. The patient had abdominal swelling, ascites with positive cytology, and elevated serum CA-125. At surgery multifocal or diffuse peritoneal involvement was found. Microscopic ovarian serosal surface involvement was demonstrated. Tumor cells stained positive for cytokeratin (AE1/AE3), epithelial membrane antigen, B72.3, Ber-EP4, Leu-M1, p53, carcinoembryonic antigen, placental alkaline phosphatase, S-100, CA-125, and estrogen receptors.
Keywords: Peritoneal carcinoma, papillary carcinoma, extraovarian neoplasms, immunohistochemistry.
RESUMEN
Se presenta un caso de carcinoma peritoneal primario extraovárico. Este raro e interesante tumor se caracteriza por una carcinomatosis peritoneal y por un patrón histológico similar al del carcinoma papilar seroso de ovario, pero sin comprometer o sólo afectando microscópicamente la superficie de los ovarios. La paciente presentaba hinchazón abdominal, ascitis con citología positiva y el CA-125 elevado en el suero. En el momento de la cirugía se encontró una afectación peritoneal difusa o multifocal, existiendo afectación microscópica de la superficie serosa ovárica. Las células tumorales fueron positivas para citoqueratina (AE1/AE3), antígeno de membrana epitelial, B72.3, Ber-EP4, Leu-M1, p53, antígeno carcinoembrionario, fosfatasa alcalina placentaria, proteína S-100, CA-125 y receptores estrogénicos.
Palabras clave: Carcinoma peritoneal, carcinoma papilar, neoplasia extraovárica, inmumohistoquímica.
INTRODUCTION
Extraovarian primary peritoneal carcinoma (EOPPC) is a rare malignant epithelial tumor that develops from the peritoneum lining the pelvis and abdomen and is characterized by abdominal carcinomatosis, uninvolved or minimally involved ovaries, and no identifiable primary tumor (1). It is similar to serous ovarian carcinoma with respect to clinical presentation, histologic appearance, pattern of spread, treatment, and prognosis (2). This entity has been reported under various names including serous surface papillary carcinoma of the peritoneum, extraovarian müllerian adenocarcinoma, multiple focal extraovarian serous carcinoma, and normal-sized ovary carcinoma syndrome (2,3). This entity was first described by Swerdlow in 1959 as «mesothelioma of the pelvic peritoneum» (4). Although most cases of EOPPC are of serous histology, nonserous tumors have been reported. It accounts for between 7% and 15% of cases with a diagnosis of ovarian cancer (2).
CASE REPORT
A-73-year-old woman presented with a 4-month history of lower abdominal pain, swelling, and weight loss. Gynecological and medical history were uneventful. On physical examination, a large ill defined anterior abdominal mass, was found. No other masses were detected anywhere else. Liver and spleen were not palpable, and the gastrointestinal examination was negative. No lymph nodes were palpable. Examination of the genitourinary system was negative. Laboratory data showed that serum CA-125 value was significantly elevated. Abdominopelvic computed tomography (CT) scan showed ascites, and an omental caking (diffuse enlargement) of low density within the peritoneal cavity (fig. 1A). An exploratory laparotomy revealed several small tumor nodules on the omentum that became matted to form a «tumor cake» up to 15 cm in maximal dimension, hemorrhagic ascites, diffuse abdominopelvic tumor implants described as granular over the parietal peritoneum, and on the visceral peritoneum of the large bowel. Minute superficial excrescences were also seen on the surface of the ovaries, but retain their normal size and shape. A biopsy of the omentum showed infiltrating papillary adenocarcinoma. Following surgery, she received six courses of chemotherapy during a 6-month period. A second-look laparotomy was performed six months after the first surgery. Total hysterectomy, bilateral salpingo-oophorectomy, and omentectomy were performed. The diaphragm, liver surface, gallbladder, pancreas, stomach, intestines, and kidneys were all palpated and appeared normal and free of disease. There were no enlarged or palpable pelvic or paraaortic lymph nodes or other retroperitoneal pathology. The uterus, tubes, and ovaries were all normal in size and gross appearance. Nodular infiltration of the omentum was present (fig. 1B). Histological examination showed a poorly differentiated papillary serous carcinoma on the omentum, and on the surface of the bilateral ovaries, but no primary site was found anywhere. Her postoperative recovery was uneventful. Three months after diagnosis, she remains in complete remission with a negative computed tomography scan of the abdomen and pelvis, and a normal CA-125.
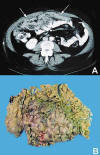
Fig. 1:
(A) CT scan showing a diffuse omental enlargement
(arrows). (B) The omentum was partly replaced with tumor nodules.
Materials and methods
The excised specimen was fixed in 10% buffered formalin, embedded in paraffin, cut in 4m sections and stained with hematoxylin and eosin. Sections were studied immunohistochemically with automated immunostaining on a Biotek Solutions Tech Mate (TechMate 500; Biotech Solutions, Dako, Glostrup, Denmark) and then incubated in a detection Kit (CheMate, code K4001, Dako) according to the manufacturer’s instructions. Peroxidase activity was developed with 3-3’-diaminobenzidine (Sigma Chemical Co.) to obtain a brown end product. Representative sections were examined by using positive and negative controls. The following commercially available antibodies were employed: epithelial membrane antigen (EMA) (E29, 1:100), B72.3 (B72.3, prediluted) (Biogenex, San Ramon, CA, USA), cytokeratin (AE1/AE3, 1:20), calretinin (5A5, 1:100), vimentin (V9, 1:200), p53 (DO-7, 1:100), estrogen receptors (6F11, 1:50), CA19-9 (C241, 1:200) (Novocastra, Newcastle, UK), Leu-M1 (C3D-1, 1:50),carcinoembryonic antigen (CEA) (II-7, 1:100), placental alkaline phosphatase (PLAP) (8A9, 1:50) CA-125 (M11, 1:20), thrombomodulin (1009, 1:100), cytokeratin 5/6 (CK5/6.007, 1:25), epithelial antigen (Ber-EP4, 1:40) and protein S-100 (S-100, 1:40) (Dako, Carpenteria, CA, USA).
Pathological findings
Microscopically the tumor was arranged in clusters and in small nests separated by desmoplastic tissue (fig. 2A), containing a few lymphocytes and psammoma bodies scattered throught the tumor (fig. 2B). In some areas, a cystic growth pattern with intracystic tufting, and small aggregations of papillary clusters were present (fig. 2C). The papillary structures were lined by several layers of cells with nuclear crowding and a high nuclear-cytoplasmic ratio (fig. 3A). The tumor cells were polyhedral with indistinct cell borders and finely granular eosinophilic cytoplasm. Cytologically, nuclei were of high grade with vesicular chromatin and prominent nucleoli (fig. 3B), and showed frequent mitoses (5/10 high-power fields). The tumor had infiltrated the omentum and also the surface of the ovaries involving only ovarian surface epithelium.
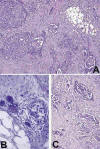
Fig. 2:
(A) Tumor contains a mixture of patterns, with
solid and complex glandular structures, which are partly papillary. A
desmoplastic response is prominent (HE, 100x). (B) A few scattered lymphocytes
and psammoma bodies are present (HE, 400x). (C) Area with papillary formations
of tumor cells (HE, 200x).

Fig. 3:
(A) Micropapillary structures of tumor cells
projecting into the lumen of a microcyst (HE, 400x). (B)Tumor cells are large,
with indistinct cell borders, cellular and nuclear pleomorphism and visible
nucleoli (HE, 1000x).
Staining for cytokeratin (AE1/AE3), and Leu-M1 was diffuse and cytoplasmic in location. The pattern of the reaction for Ber-EP4, and CA-125 was purely cell membrane-based. The tumor cells showed intense nuclear p53 and estrogen receptors (ER) staining. B72.3, EMA, and PLAP demonstrated predominantly cell membranous localization with weak cytoplasmic labeling. S-100 protein was expressed in the nuclei and cytoplasm of positive tumor cells, and CEA and CA19-9 expression was observed with weak and focal cytoplasmic staining (fig. 4). The tumor cells were consistently negative for calretinin, thrombomodulin, cytokeratin 5/6, and vimentin.
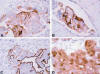
Fig. 4:
Tumor cells showing cell membranous staining with
B72.3 (A), PLAP (B), and Ber-EP4 (C). Positive S-100 staining in both the
nucleus and the cytoplasm (D) (400x).
DISCUSSION
EOPPC, a relatively newly defined disease, is a rare adenocarcinoma that arises in the peritoneum. Aproximately 3.2% to 21% of EOPPC patients have a history of bilateral oophorectomy for benign disease or prophylaxis in women with a family history of ovarian carcinoma. The age distribution and clinical presentation is indistinguishable from that of advanced-stage epithelial ovarian cancer (5). Most reported cases of EOPPC have been in women, usually elderly. However, rare cases have been reported in children (6) and males (7). Patients ordinarily present with nonspecific abdominal symptoms and ascites, reported in approximately 85% of cases (1). At laparotomy, almost all cases show diffuse peritoneal tumor implants, which usually involve the omentum and upper abdomen. Although EOPPC always involves the full thickness of the omentum, invasion into other abdominal or pelvic organs is rare and, when present, tends to be superficial. The ovaries are almost always of normal size and shape, and frequently display surface tumor implants which may be focally invasive; rarely, they may be normal grossly and microscopically (2). These findings are similar to those of advanced-stage epithelial ovarian cancer or peritoneal carcinomatosis from metastatic gastrointestinal cancers, except that the ovaries show minimal or no involvement and no primary can be found in the gastrointestinal tract or other organs (1). Identification of the correct primary site is critical because the surgical management of EOPPC is vastly different from that of carcinomatosis associated with other malignancies (5). Although EOPPC may be considered in the differential diagnosis, it is not a diagnosis that can made preoperatively. The diagnosis of EOPPC is typically made by exclusion after both operative assessment and pathological study. If ovaries seem normal with widespread disease elsewhere in the abdomen, EOPPC becomes a leading diagnostic possibility. However, because surface involvement of the ovaries is present in approximately 96% of the cases, the distinction between EOPPC and epithelial ovarian carcinoma may only be made after histological examination to evaluate the extent of ovarian invasion by tumor (5).
EOPPC spreads mainly transperitonealy; however, lymphatic and blood-borne metastases have been suggested. Metastases to different groups of lymph nodes, the liver (8), and the brain (9) have been reported.
Most cases of EOPPC reported in the literature have been of serous histology. However, other histologic variants of the müllerian system have been reported; specifically, endometrioid, clear cell, mucinous, Brenner tumor, and mixed müllerian tumors; but nonserous and serous tumors appear to be similar with regard to prognosis and response to therapy (10).
The light microscopic, histochemical, immunohistochemical, and ultrastructural features of EOPPC are similar to those of ovarian serous carcinoma. Thus, EOPPC appears as a high-grade, purely epithelial neoplasm with frequent mitotic figures, necrosis, slitlike glandular spaces, and psammoma bodies (11).
In order to differentiate EOPPC from papillary serous adenocarcinoma of the ovary, the Gynecologic Oncology Group has stipulated that the following criteria be met (2): 1) histology must be predominantly serous or identical to any grade of ovarian papillary serous tumor, 2) the ovaries are of normal size or enlarged by a benign process, 3) the involvement in the extraovarian sites must be greater than the involvement on the surface of either ovary, and 4) the ovarian component must be nonexistent, or confined to surface epithelium or less than 5 x 5 mm. within the stroma. Furthermore, some authors suggested that genetic events (HER-2/neu overexpression) responsible for malignant transformation in EOPPC may be distinct from those responsible for epithelial ovarian cancer (11).
Also, EOPPC must be differentiated from malignant mesothelioma, benign papillary mesothelioma, metastatic peritoneal carcinomatosis, borderline primary peritoneal serous tumor, endosalpingiosis, and psammocarcinoma of the peritoneum. Malignant mesothelioma is closely related to long-term exposure to asbestos, has a male predominance, frequent spindle cell component, cytoplasmic eosinophilia, and sometimes extensive cell vacuolization, and rare psammoma bodies. Previous studies have shown that the expression of B72.3, PLAP, or CEA by a papillary peritoneal tumor would militate against a diagnosis of mesothelioma; also combined reactivity for S-100 and PLAP, or S-100 and B72.3, characterizes the majority of serous adenocarcinomas, and is not observed in mesotheliomas (12). Ordoñez reported that from the practical point of view, calretinin, thrombomodulin, and keratin 5/6 are the best positive markers for distinguishing between epithelial malignant mesotheliomas and papillary serous carcinomas diffusely involving the peritoneum. Among the antibodies that are considered to be negative markers for mesothelioma, MOC-31, B72.3, Ber-EP4, CA19-9, and Leu-M1 proved to the best diagnostic discriminators (13). Other investigators have found that a two-marker panel of antibodies including vimentin and Ber-EP4 is most useful for the differential diagnosis between carcinoma and mesothelioma (14). Also, recently, Ordoñez indicate that because ER is frequently expressed in serous carcinomas but not in mesotheliomas, this marker could be very useful to discriminate between these malignancies (15). Benign papillary mesothelioma occurs in both men and women, usually of young age, has well-formed papillae, mostly lined by one layer of a single cell type that resemble reactive mesothelium, and showing little or no anaplasia or mitoses; and an absence of invasion into the peritoneum or abdominopelvic organs (16).
To the best of our knowledge, no morphologic features can afford a reliable distinction between EOPPC and metastatic peritoneal carcinomatosis; diagnosis of the latter rests on recognizing a primary tumor, usually in the ovary, fallopian tube, or endometrium and less frequently in other organs such as breast, gastrointestinal tract (especially stomach, pancreas), lungs, and thyroid gland (11).
Primary serous borderline tumors of the peritoneum have also been reported, albeit in fewer numbers than carcinomas, affecting younger patients, and having the microscopic features of ovarian borderline serous tumor. These primary serous borderline tumors have an excellent prognosis, although rare cases have been reported in which transformation to carcinoma has been observed on follow-up examination (17). Endosalpingiosis is a benign lesion, found most often in association with chronic salpingitis; it is most commonly encountered in the pelvic peritoneum but rarely involves other portions of the peritoneum, and consists of glandular inclusions lined by normal-appearing tubal-type epithelium (18).
Also, another less virulent variation of EOPPC is discussed in the literature (19). This is the serous psammocarcinoma of the peritoneum, has a proportionately larger number of psammoma bodies, and a less-aggressive cytologic appearance with absent or, at most, moderate nuclear atypia and rare mitotic figures.
The pathogenesis of EOPPC has been controversial. Some authors believe that embryonic germ cell rests remain along the gonadal embryonic pathway and that EOPPC develops from a malignant transformation of these cells (12). Other authors contend that field carcinogenesis occurs, with the celomic epithelium lining the abdominal cavity (peritoneum) and the ovaries (germinal epithelium) manifesting a common response to an oncogenic stimulus (18). Muto et al. have suggested a multifocal origin with clonality studies (21). However, Kupryjanczyk et al. have identified others findings that are consistent with a unifocal origin (22). Therefore, more extensive studies of this type should be performed to confirm these results.
Recently, Euscher et al. (23) investigated the WT-1 expression in serous carcinoma arising from different sites within the female genital tract and suggested that serous carcinoma may have a different biology based on site of origin.
The prognosis of EOPPC is poor. Medial survival time vary between 7 and 27.8 months, while 5-year survival rates range from 0% to 26.5% (8).
Treatment usually includes abdominal hysterectomy, bilateral salpingo-oophorectomy, and tumor debulking followed by chemotherapy. Surgery remains critically important for both the diagnosis and the therapy of EOPPC. Once the diagnosis has been established and the extent of disease documented, maximal cytoreduction becomes the primary goal of the procedure. Excision of all visible implants is the hallmark of cytoreductive efforts. The subsequent courses include, in most cases, initial good response followed months lather by uncontrollable, lethal local recurrence; rapid and lethal progression of local disease without any evidence of initial response to chemotherapy; and, in rare case, long-term maintenance of the initial response, with the patient being considered cured (5).
REFERENCES
Eltabbakh GH, Piver MS. Extraovarian primary peritoneal carcinoma. Oncology (Willinston Park) 1998; 12: 813-25.
Bloss JD, Liao SY, Buller RE, Manett A, Berman ML, McMeekin S, et al. Extraovarian peritoneal serous papillary carcinoma: a case-control retrospective comparison to papillary adenocarcinoma of the ovary. Gynecol Oncol 1993; 50: 347-51.
Feuer GA, Shevchuk M, Calanog A. Normal-sized ovary carcinoma syndrome. Obstet Gynecol 1989; 73: 786-92.
Swerdlow M. Mesothelioma of the pelvic peritoneum resembling papillary cystadenocarcinoma of the ovary. Am J Obstet Gynecol 1959; 77: 197-200.
Chu Cs, Menzin AW, Leonard DGB, Rubin SC, Wheeler JE. Primary peritoneal carcinoma: A review of the literature. Obstet Gynecol Surv 1999; 54: 323-35.
Shibata R, Matsufuji H, Morimoto T, Hosoya R, Araki K, Hata J. Extraovarian primary peritoneal carcinoma in a child. Pediatr Blood Cancer 2004; 42: 292-3.
Shmueli E, Leider-Trejo L, Schwartz I, Aderka D, Inbar M. Primary papillary serous carcinoma of the peritoneum in a man. Ann Oncol 2001; 12: 563-7.
Fromm GL, Gershenson DM, Silva EG. Papillary serous carcinoma of the peritoneum. Obstet Gynecol 1990; 75: 75-89.
Eltabbakh GH, Piver MS, Werness BA. Primary peritoneal adenocarcinoma metastatic to the brain. Gynecol Oncol 1997; 66: 160-3.
Altaras MM, Aviram R, Cohen I, Cordoba M, Weiss E, Beyth Y. Primary peritoneal papillary serous adenocarcinoma: clinical and management aspect. Gynecol Oncol 1991; 40: 230-6.
Mills SE, Andersen WA, Fechner RE, Austin MB. Serous surface papillary carcinoma. A clinicopathologic study of 10 cases and comparison with stage III-IV ovarian serous carcinoma. Am J Surg Pathol 1998; 12: 827-34.
Bollinger DJ, Wick MR, Dehner LP, Mills SE, Swanson PE, Clarke RE. Peritoneal malignant mesothelioma versus serous papillary adenocarcinoma. Am J Surg Pathol 1989; 13: 659-70.
Ordoñez NG. Role of immunohistochemistry in distinguishing epithelial peritoneal mesotheliomas from peritoneal and ovarian serous carcinomas. Am J Surg Pathol 1998; 22: 1203-14.
García-Prats MD, Ballestin C, Sotelo T, Lopez-Encuentra A, Mayordomo JI. A comparative evaluation of immunohistochemical markers for the differential diagnosis of malignant pleural tumours. Histopathology 1998; 32:462-72.
Ordoñez NG. Value of estrogen and progesterone receptor immunostaining in distinguishing between peritoneal mesotheliomas and serous carcinomas. Hum Pathol 2005; 36:1163-7.
Goepel JR. Benign papillary mesothelioma of peritoneum: A histological, histochemical and ultrastructural study of six cases. Histopathology 1987; 5: 21-30.
Bell DA, Scully RE. Serous borderline tumors of the peritoneum. Am J Surg Pathol 1990; 14: 230-49.
Zinser KR, Wheeler JE. Endosalpingiosis in the omentum. A study of autopsy and surgical material. Am J Surg Pathol 1982; 6: 109-17.
Gilks CB, Bell DA, Scully RE. Serous psammocarcinoma of the ovary and peritoneum. Int J Gynecol Pathol 1990; 9: 110-21.
Kannerstein M, Churg J. Peritoneal mesothelioma. Hum Pathol 1997; 8: 83-94.
Muto MG, Welh WR, Mok SC, Bandera CA, Fishbaugh PM, Tsao SW, et al. Evidence for a multifocal origin of papillary serous carcinoma of the peritoneum. Cancer Res 1995; 55: 490-2.
Kupryjanczyk J, Thor AD, Beauchamp R, Poremba C, Scully RE, Yandell DW. Ovarian, peritoneal, and endometrial serous carcinoma: Clonal origin of multifocal disease. Mod Pathol 1996; 9: 166-73.
Euscher ED, Malpica A, Deavers MT, Silva EG. Differential expression of WT-1 in serous carcinomas in the peritoneum with or without associated serous carcinoma in endometrial polyps. Am J Surg Pathol 2005; 29: 1074-8.